
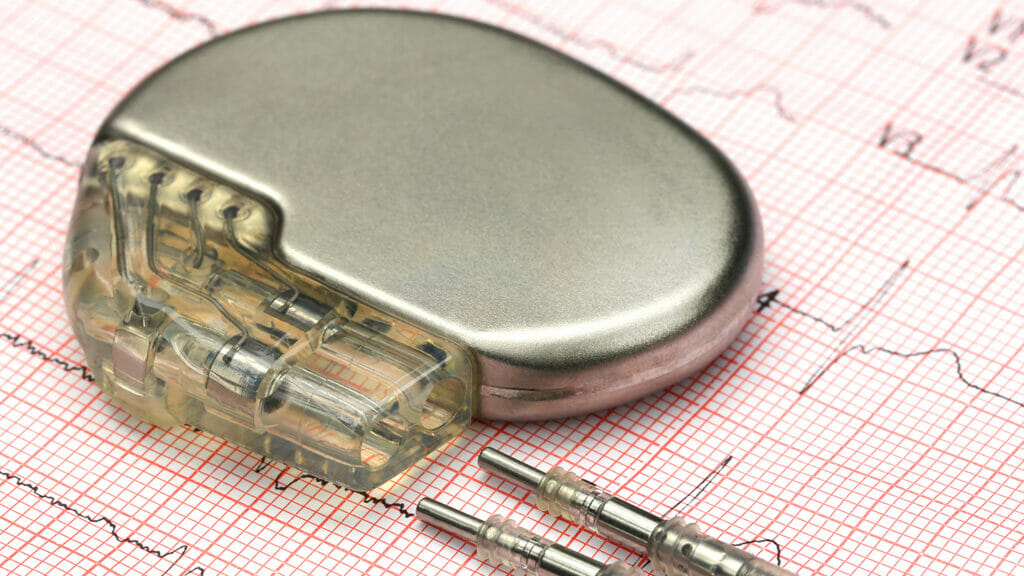
Makers of a newly certified pacemaker model tout the device’s innovative functions that automate processes including post-discharge inspections and therapy for stable heart rhythms.
The Amvia Edge pacemaker received approval from the US Food and Drug Administration, creator Biotronik has announced.
Managing the care of residents with pacemakers is a major challenge for assisted living communities and other long-term care operators; 70% of the 3 million Americans with pacemakers are aged 65 or more years.
The greatest benefit of the Amvia Edge, the company states, is its “MR Guard” technology, which allows the pacemaker to automatically switch to a different mode during MRI scans, a process that typically requires additional visits and programming.
In addition, the Amvia Edge has capabilities that allow for fast reporting of conditions, both immediately after implantation of the device, and as part of routine monitoring, allowing for a more efficient evaluation of patients, the company stated.
“The clinical treatment options and built-in efficiencies of Amvia are designed to solve everyday challenges for patients, physicians and caregivers,” BIOTRONIK President Ryan Walters said in a statement last week.
Although the Amvia Edge touts its improved, automated, functions, other new pacemakers are being developed to improve overall design, the McKnight’s Clinical Daily has reported.
In particular, a leadless pacemaker from Abbott received FDA approval last month.
Although cardiac rehabilitation and exercise are important ways to improve quality of life for those with heart disease, facilities can take steps to improve their quality of care overall for residents and patients with heart conditions, experts point out. One specific example recommends updating standards for nursing facilities and defibrillator use in CPR emergencies.


